Lecture 11: Electrochemical Techniques
[Previous Lecture] [Next Lecture] [Course Outline] [General Info] [Study Guide]
Polarisation Curves of Passivating Metals
From previous lectures and tutorials we have observed that for some metals/alloys, when polarisation (anodic) increases, the current density and hence the corrosion rate of the metal/alloy increases; while for others, the corrosion rate will drastically decrease to a stable value and become independent of the polarisation applied. This behavior is referred to as passivating behavior and the metal/environment is referred to as a passivating system. Polarisation curves are particularly valuable in quantifying the behaviour of materials under various conditions. This section explains how electrochemical techniques are used to assess materials resistance to corrosion and what information can be obtained.

The schematic potentiodynamic polarisation behaviour of passivating
metals.
(a) General polarisation plot showing three possible theoretical cathode processes.
The solid line in Fig. 7.6(a) is a schematic E/lg i plot for a metal in a mild environment, such as an austenitic stainless steel in dilute sulphuric acid.
- AB represents cathodic behaviour,
- BG is the active zone. The metal is not passivated at its free corrosion potential, B.
- AC and DC are Tafel-type straight lines drawn for the reduction and oxidation reactions
of the normal metal dissolution (M=M++e).
- At potentials more positive than B, corrosion rate increases, and reaches a maximum at the passivation potential, G, which is often given the symbol, Epp.
- The transition from active dissolution occurs as a solid species becomes more thermodynamically stable than the metal ion. A protective film begins to form and causes a sudden drop in corrosion current density in the region G to J.
- From J to P, the passive zone, the current density is maintained at a steady, low level, until,
- at P, breakdown of the protective film begins. It is here that the likelihood of pitting is greatest, and consequently
- the potential Ec, often called the critical pitting or breakdown potential. It is a useful parameter in assessing pitting properties of materials. It should be noted that it is not an absolute parameter, and varies according to both metallurgical and environmental conditions.
- At potentials more positive than P, the current density begins to rise as more and more pits propagate.
Unstable Passivation
Some passivating metals have E/lg i plots which may show more than one corrosion potentials like in Fig.7.6(b). This indicates an unstable passivating system.
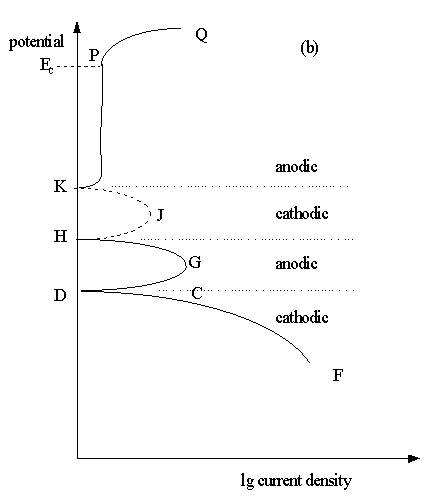
Fig.7.6(b) The schematic potentiodynamic polarisation behaviour of passivating metals.
These differences are caused by variation in the dominant cathodic process. Three possibilities are shown as dashed lines, CA, KF, and LN in Fig. 7.6(a). A different type of polarisation plot will be measured, depending upon which cathodic process predominates.
- There are three usual cathodic processes:
- hydrogen evolution,
- oxygen reduction and
- metal cation reduction.
- The experimental plot is always the sum of all anodic and cathodic currents.
Cyclic Pitting Scan and Pitting Potential
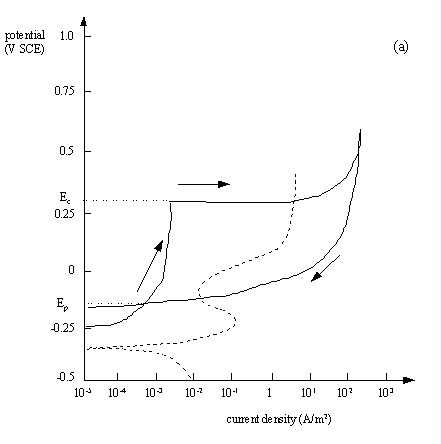
Fig.7.8(a) A cyclic polarisation (pitting 'hysteresis') curve for type 304 stainless steel in aerated seawater at 25 oC, scan rate: 0.1 mV s-1. The broken line shows a similar plot using aerated seawater + 4.5 M sodium chloride and HCl, adjusted to pH 1.2.
- Ec is the critical pitting potential above which new pits will initiate and existing pits will propagate
- Ep is the protection potential below which there is no pitting
- Ep<E<Ec between the two, existing pits will propagate but no new pits will form
In general, the larger the area of the hysteresis loop, the greater the susceptibility to pitting corrosion.
Applications of Electrochemical Techniques
Electrochemical techniques can be used to ASSESS materials resistance to
- general corrosion
- crevice corrosion
- pitting corrosion
- intergranular cracking
HOW ???
Potentiodynamic polarisation measurement: the E-lgi plot for information on
- pitting potential
- critical pitting potential
- protection potential
- breakdown potential
- critical pitting temperature
- active region
- passive region
- transpassive region
Summary
A corroding system can be classified as passive or non-passive system depending on the behaviour under anodic polarisation. Polarisation curves for the passive systems may show active/passive and/or passive/transpassive transitions. The current density in the passive state is generally very low compared with that in the active state. The stability of passivation depends on the predominant cathodic process (hydrogen evolution, reduction oxygen and reduction metal ions). Electrochemical techniques provide rapid, accurate and quantitative evaluation of a materials resistance to corrosion, particularly pitting. The critical pitting potential and the protection potential can be obtained from a cyclic pitting scan.
To reinforce learnings in this lecture read pages 172-177
(textbook)
To prepare yourself for the next lecture
read pages 191-197, 214-228 (textbook)
Copyright © 1995-2025.. All rights reserved.